Abstract

Background
Prognostic scoring system has made a progress to better predict progression and survival of diffuse large B-cell lymphoma (DLBCL) patients. International prognostic index (IPI) is still valid even after the advent of rituximab for the treatment of DLBCL, however, there is still need for sophisticated prognostic scoring system with simply testable variables to individualize prognosis in patients treated with immunochemotherapy. In this context, various inflammatory factors detected by complete blood count and chemistry have been evaluated in several studies: the white blood cell count, the absolute lymphocyte count (ALC), the absolute monocyte count (AMC), the platelet-to-lymphocyte ratio, the neutrophil-to-lymphocyte ratio, the lymphocyte-to-monocyte ratio, lactate dehydrogenease (LDH), albumin, C-reactive protein (CRP), ferritin, and beta 2-microglobulin. This study aimed to make an inflammatory factor based scoring system for predicting survival and disease progression in DLBCL patients undergoing rituximab-based chemotherapy and estimate the possibility of elaboration of IPI with inflammatory factor based scoring system.
Method
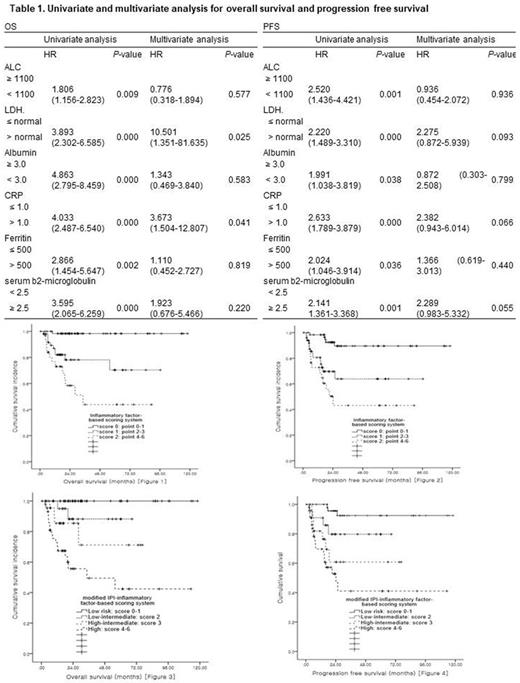
Five hundred and twenty-three patients with DLBCL treated with at least 2 or more cycles of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) as a first-line treatment were retrospectively analyzed. Each 6 factors for inflammatory scoring system were dichotomized by the reference of previously published papers and were given 1 point for each variables after the following criteria: the ALC < 1100/mm3, LDH > normal, albumin < 3.0 g/dL, CRP > 1.0 mg/dL, ferritin > 500 ng/mL, and beta 2-microglobulin ≥ 2.5 mg/L. Patients were further classified by inflammatory factor-based scoring system: score 0 (points 0-1), score 1 (points 2-3) and score 2 (points 4-6) and these scores substituted LDH in the IPI. The IPI scores plus scores by inflammatory factor-based scoring system was further classified into 4 risk groups: low (score 0-1), low-intermediate (score 2), high-intermediate (score 3), and high (score 4-6).
Result
The median follow-up duration was 37.61 months (range 0.60 - 139.03 months). All of the variables including the ALC < 1100/mm3, LDH > normal, albumin < 3.0 g/dL, CRP > 1.0 mg/dL, ferritin > 500 ng/mL, and beta 2-microglobulin ≥ 2.5 mg/L were statistically significant for progression free survival(PFS) and (OS) by univariate analysis. In multivariate analysis, LDH > normal and CRP > 1.0 mg/dL remained statistically significant for OS and CRP > 1.0 mg/dL was marginally significant for PFS (Table 1). By inflammatory factor-scoring system, score 0, 1, and 2 showed statistically significant OS (Figure 1, P= 0.000) and PFS (Figure 2, P= 0.000), and modified IPI risk groups showed significant differences in OS (Figure 3, P= 0.000) and PFS (Figure 4, P= 0.000).
Conclusion
The risk groups stratified by inflammatory factor-based scoring system showed significant difference in both progression-free and overall survival in DLBCL patients in the Rituximab era. The inflammatory factor-based scoring system can further make delicate modification to IPI, substituting LDH. Validation of the finding in a larger cohort of patients is needed.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal